43 cautionary and advisory labels for dispensed medicines
Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; How to use BNF Publications online | About | BNF | NICE Details of these labels can be found in Guidance for cautionary and advisory labels. As these labels have now been applied at the level of the dose form, a full list of medicinal products with their relevant labels would be extensive. This list has therefore been removed, but the information is retained within the monograph.
Poisons Standard June 2021 - Legislation 26.05.2021 · Schedule 1. This Schedule is intentionally blank. Schedule 2. Pharmacy Medicine – Substances, the safe use of which may require advice from a pharmacist and which should be available from a pharmacy or, where a pharmacy service is not available, from a licensed person.. Schedule 3. Pharmacist Only Medicine – Substances, the safe use of which requires …
Cautionary and advisory labels for dispensed medicines
R.A. 7394 - Lawphil 2) Any drug dispensed by filling or refilling a written prescription of a practitioner licensed by law to administer such drug shall be exempt from the requirements of Article 89, except paragraphs (a), (h), (2) and (3), and the packaging requirements of paragraphs (f) and (g), if the drug bears a label containing the name and address of the dispenser, the serial number and the date of the ... Chapter 400j - Pharmacy - Connecticut General Assembly (17) to ban advertising or promotion of legend drugs rather than of those bearing cautionary label and made ban more forceful by adding “directly or indirectly, by any means, in any form”; P.A. 75-95 deleted former Subdiv. (17) banning advertising and renumbered remaining Subdivs.; P.A. 76-166 revised Subdiv. (8) banning drug substitution except as provided in Secs. 20-185b and 20 … [JDK-8141210] Very slow loading of JavaScript file with ... FULL PRODUCT VERSION : java version "1.8.0_66" Java(TM) SE Runtime Environment (build 1.8.0_66-b17) Java HotSpot(TM) 64-Bit Server VM (build 25.66-b17, mixed mode ...
Cautionary and advisory labels for dispensed medicines. Could Call of Duty doom the Activision Blizzard deal? - Protocol Oct 14, 2022 · Hello, and welcome to Protocol Entertainment, your guide to the business of the gaming and media industries. This Friday, we’re taking a look at Microsoft and Sony’s increasingly bitter feud over Call of Duty and whether U.K. regulators are leaning toward torpedoing the Activision Blizzard deal. Guidance for cautionary and advisory labels | About | BNF | NICE Wordings which can be given as separate warnings are labels 1–19, 29–30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21–28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted. [JDK-8141210] Very slow loading of JavaScript file with ... FULL PRODUCT VERSION : java version "1.8.0_66" Java(TM) SE Runtime Environment (build 1.8.0_66-b17) Java HotSpot(TM) 64-Bit Server VM (build 25.66-b17, mixed mode ... Chapter 400j - Pharmacy - Connecticut General Assembly (17) to ban advertising or promotion of legend drugs rather than of those bearing cautionary label and made ban more forceful by adding “directly or indirectly, by any means, in any form”; P.A. 75-95 deleted former Subdiv. (17) banning advertising and renumbered remaining Subdivs.; P.A. 76-166 revised Subdiv. (8) banning drug substitution except as provided in Secs. 20-185b and 20 …
R.A. 7394 - Lawphil 2) Any drug dispensed by filling or refilling a written prescription of a practitioner licensed by law to administer such drug shall be exempt from the requirements of Article 89, except paragraphs (a), (h), (2) and (3), and the packaging requirements of paragraphs (f) and (g), if the drug bears a label containing the name and address of the dispenser, the serial number and the date of the ...














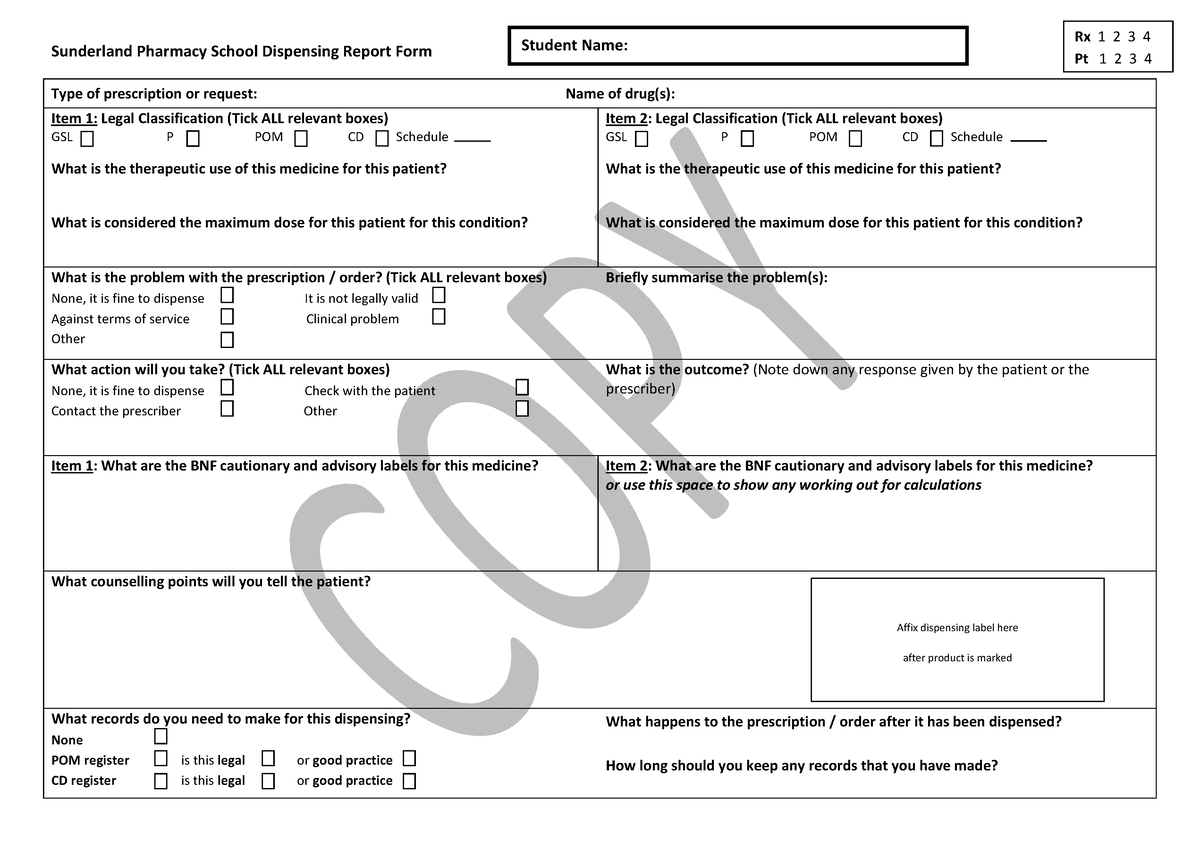








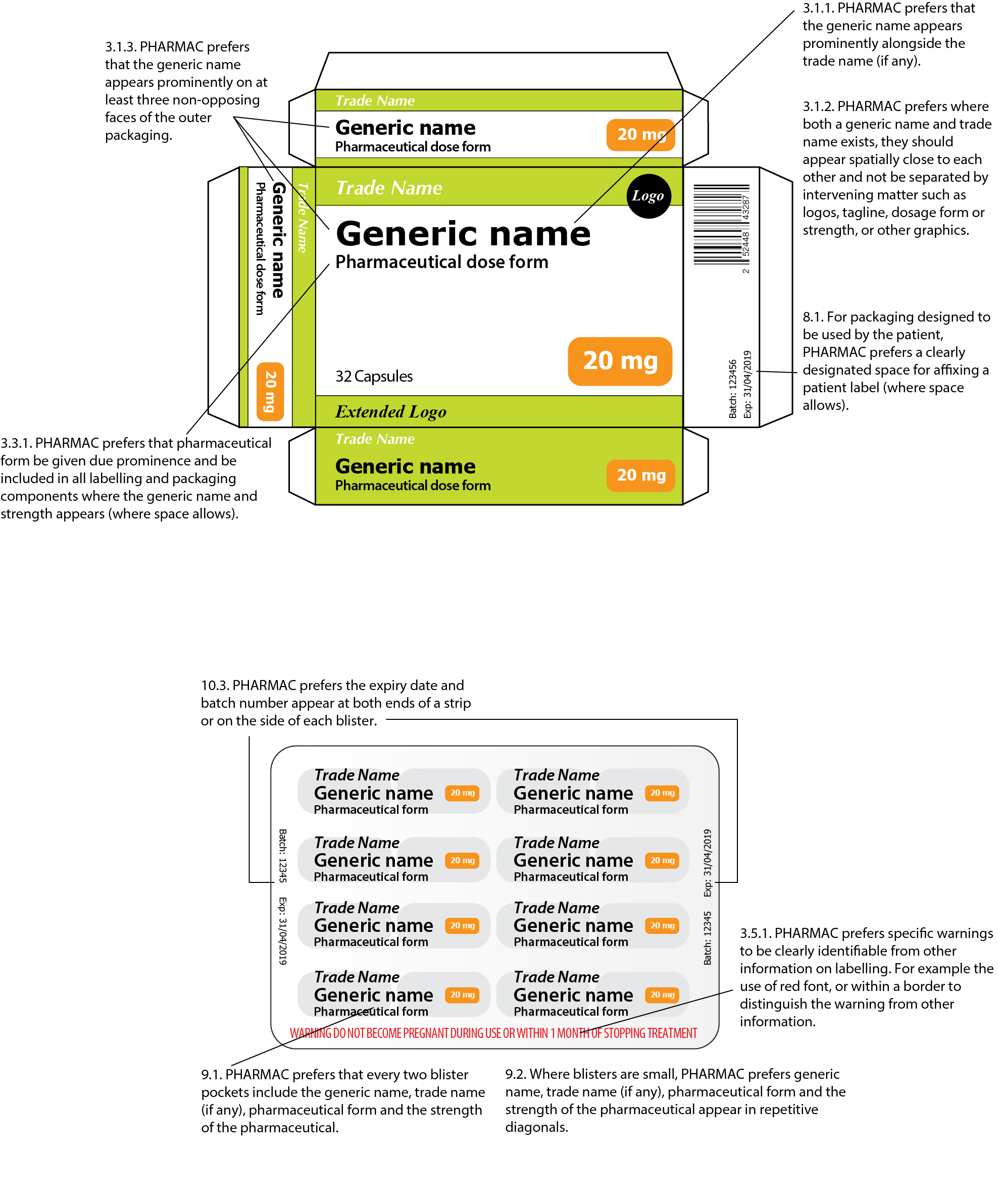








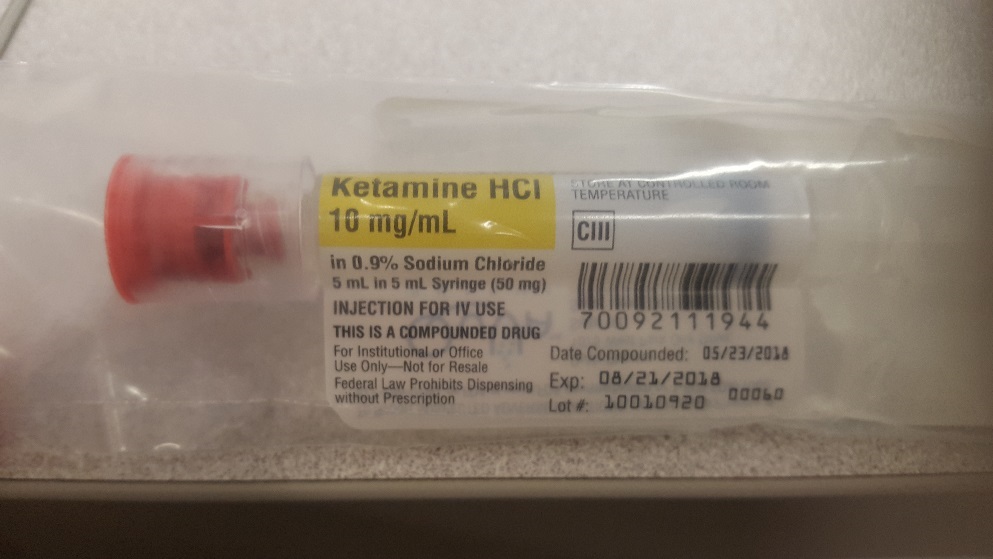



Post a Comment for "43 cautionary and advisory labels for dispensed medicines"